In particular, contributions made by Lothar Meyer and Dmitri Mendeleev made trends in element properties apparent. They independently proposed Periodic Law in 1869. The periodic table arranged the elements to reflect Periodic Law, even though scientists at the time had no explanation for why properties followed a trend. The classic Periodic Table organizes the chemical elements according to the number of protons that each has in its atomic nucleus.
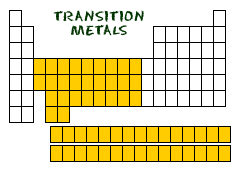
The largest family of elements consists of transition metals. The center of the periodic table contains the transition metals, plus the two rows below the body of the table (lanthanides and actinides) are special transition metals.
The periodic table of elements arranges all of the known chemical elements in an informative array. Elements are arranged from left to right and top to bottom in order of increasing atomic number. Order generally coincides with increasing atomic mass. In the periodic table of the elements, each numbered column is a group. Periodic Law was formulated based on observations made by scientists in the 19th century.
Lithium and sodium both have a +1 oxidation state and are reactive, shiny metals. Another way to group elements is based on their shared properties (in some cases, these groupings do not correspond to the columns in the periodic table).
What Is a Periodic Sentence? (with Examples)
Element families are indicated by numbers located at the top of the periodic table. “The constitution of group 3 of the periodic table”.
The metalloids have intermediate properties. Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period.
The periodic table is a chart used in chemistry that shows chemical elements in order of their atomic number. Periodicity was helpful to Mendeleev because it showed him gaps in his periodic table where elements should be.
After writing the properties of the elements on cards, he began ordering them by increasing atomic weight, according to the Royal Society of Chemistry. This is when he noticed certain types of elements regularly appearing. After intensely working on this “puzzle” for three days, Mendeleev said that he had a dream in which all of the elements fell into place as required.
The IUPAC proposal was first circulated in 1985 for public comments, and was later included as part of the 1990 edition of the Nomenclature of Inorganic Chemistry. In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements.
There are three systems of group numbering for the groups; the same number may be assigned to different groups depending on the system being used. The modern numbering system of “group 1” to “group 18” has been recommended by the International Union of Pure and Applied Chemistry (IUPAC) since about 1990. It replaces two older incompatible naming schemes, used by the Chemical Abstract Service (CAS, more popular in the US), and by IUPAC before 1990 (more popular in Europe). The system of eighteen groups is generally accepted by the chemistry community, but some dissent exists about membership of several elements. Disagreements mostly involve elements number 1 and 2 (hydrogen and helium), as well as inner transition metals.
Synonyms of ’periodic’
Within this classification system, hydrogen is a nonmetal. The nonmetals, halogens, and noble gases are all types of nonmetallic elements.
There are 18 numbered groups in the periodic table; the f-block columns (between groups 3 and 4) are not numbered. Dmitri Mendeleev, a Russian chemist and inventor, is considered the “father” of the periodic table, according to the Royal Society of Chemistry. In the 1860s, Mendeleev was a popular lecturer at a university in St. Petersburg, Russia. Since there were no modern organic chemistry textbooks in Russian at that time, Mendeleev decided to write one, and simultaneously tackle the problem of the disordered elements.
- After writing the properties of the elements on cards, he began ordering them by increasing atomic weight, according to the Royal Society of Chemistry.
- Mendeleev ultimately wrote the definitive chemistry textbook of his time, titled “Principles of Chemistry” (two volumes, 1868–1870), according to Khan Academy.
- As he was working on it, he came upon a significant discovery that would contribute greatly to the development of the current periodic table.
This helped scientists find new elements because they could be expected to display certain characteristics based on the location they would take in the periodic table. Now that the elements have been discovered, scientists and students used periodicity to make predictions about how elements will behave in chemical reactions and their physical properties. Periodicity helps chemists predict how the new, superheavy elements might look and behave. Mendeleev organized elements according to recurring properties to make a periodic table of elements. Elements within a group (column) display similar characteristics.

Periodic Sentence
Later , Mendeleev accepted the evidence for their existence, and they could be placed in a new “group 0”, consistently and without breaking the periodic table principle. A table in which the chemical elements are arranged in order of increasing atomic number. Elements with similar properties are arranged in the same column (called a group), and elements with the same number of electron shells are arranged in the same row (called a period).
The highlighted elements of this periodic table belong to the noble gas element family. The highlighted elements of this periodic table belong to the halogen element family. The highlighted elements of this periodic table belong to the alkaline earth element family. The modern IUPAC system uses Arabic numbers 1-18, simply numbering the columns of the periodic table from left to right. The older IUPAC system used Roman numerals together with letters to distinguish between the left (A) and right (B) side of the periodic table.
What does it mean if something is periodic?
The definition of periodic is something that is recurring at regular intervals, or happens from time to time. An example of periodic is a person’s birthday happening once each year.
periodic
The rows in the periodic table (the periods) reflect the filling of electrons shells around the nucleus, so when a new row begins, the elements stack on top of each other with similar properties. For example, helium and neon are both fairly unreactive gases that glow when an electric current is passed through them.
The highlighted elements of this periodic table belong to the transition metal element family. The lanthanide and actinide series below the body of the periodic table are transition metals, too. The highlighted elements of the periodic table belong to the alkali metal element family. The period number of an element signifies the highest energy level an electron in that element occupies (in the unexcited state), according to the Los Alamos National Laboratory. Group 18, the noble gases, were not discovered at the time of Mendeleev’s original table.
Mendeleev arranged the elements according to both atomic weight and valence. Not only did he leave space for elements not yet discovered, but he predicted the properties of five of these elements and their compounds. In 1869, he presented the findings to the Russian Chemical Society. His new periodic system was published in the German chemistry periodical Zeitschrift fϋr Chemie (Journal of Chemistry).
Mendeleev ultimately wrote the definitive chemistry textbook of his time, titled “Principles of Chemistry” (two volumes, 1868–1870), according to Khan Academy. As he was working on it, he came upon a significant discovery that would contribute greatly to the development of the current periodic table.
Periodic Law led to the development of the modern periodic table. The Periodic Law states that the physical and chemical properties of the elements recur in a systematic and predictable way when the elements are arranged in order of increasing atomic number. An organized table of chemical elements such as oxygen and carbon is an example of the periodic table. In the context of chemistry and the periodic table, periodicity refers to trends or recurring variations in element properties with increasing atomic number. Periodicity is caused by regular and predictable variations in element atomic structure.
Periodic Law is considered to be one of the most important concepts in chemistry. Every chemist makes use of Periodic Law, whether consciously or not, when dealing with the chemical elements, their properties, and their chemical reactions.
When he woke up, he immediately wrote them down on a piece of paper — only in one place did a correction seem necessary, he later said. The old IUPAC system was frequently used in Europe, while the CAS is most common in America. The new IUPAC scheme was developed to replace both systems as they confusingly used the same names to mean different things. The new system simply numbers the groups increasingly from left to right on the standard periodic table.